Abstract
Background: Primary central nervous system lymphoma (PCNSL) and primary testicular lymphoma (PTL) are rare mature B-cell lymphoproliferative diseases that arise at immunological sanctuary sites. Cytomorphology (CM) and flow cytometry (FCM) analysis of spinal fluid is diagnostic in about 15% of PCNSL. In the remaining patients, a tumor biopsy is necessary, which carries the risk of complications. Response assessment in PCNSL and PTL relies on imaging studies. However, MRI scans and 18F-FDG PET-scans often cannot differentiate between active tumor and scar tissue. Hence, there is a need for non-invasive tools to aid in diagnosis and help in response assessment. PCNSL and PTL are characterized by a high incidence of hotspot mutations in MYD88 and CD79B. Detection of these mutations in cell free DNA (cfDNA) might aid in diagnosis and follow-up of disease activity in patients with PCNSL and PTL. Digital droplet PCR (ddPCR) offers a robust inexpensive tool with a fast turnaround time allowing sensitive detection of such hotspot mutations.
Methods: Plasma was available for a total of 26 patients (20 PCNSL and 6 PTL) in the OncoLifes biobank of the University Medical Center Groningen. All patients gave informed consent. Corresponding tumor samples were available for 17 cases (14 PCNSL and 3 PTL). A ddPCR was performed for MYD88 L265P and in case of sufficient cfDNA also for CD79B Y196X (Bio-Rad Laboratories, Hercules, USA). The ddPCR Assay for CD79B Y196X targets the mutation Y196H c.586T>C, Y196D c.586T>G, Y196S c.587A>C, Y196C c.587A>G, and Y196F c.587A>T. The cut off value for the allele frequency (AF) used was ≥0.5%.
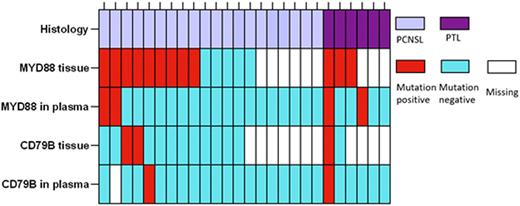
Results: The MYD88 L265P and CD79B Y196X mutations were detected in 9/14 (64.2%) and 2/13 (15.4%) of the PCNSL tissue samples. The two CD79B Y196X positive tissue cases had a concomitant MYD88 L256P. The MYD88 L265P mutation was detected in 2/20 (10%) cfDNA samples. The CD79B Y196X mutation was detected in 1/19 (5.2%) cfDNA samples; remarkably, this mutation was not detected in the corresponding tissue sample. In PTL, MYD88 L265P mutations were detected in 3/3 of tumor tissues and a CD79B Y196X mutation was detected in 1/2 of tumor tissues, which co-occurred with the MYD88 L265P. In PTL, 2/6 (33.3%) and 1/6 (16.6%) cfDNA samples were positive for MYD88 L265P and CD79B Y196X. Overall, hotspot MYD88 and/or CD79B mutations were detected in cfDNA in 5 /26 cases (18.4%) (Figure 1).
Figure 1. ddPCR results of MYD88 and CD79BConclusion: We explored the feasibility of using a combination of two common hotspot mutations in MYD88 and CD79B in PCNSL and PTL plasma samples to aid in diagnosis and disease monitoring. The data presented show that the value of ddPCR for hotspot mutations in plasma of PCNSL and PTL patients is limited.
Disclosures
Diepstra:Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding. Nijland:Roche: Research Funding; Takeda: Research Funding; Genmab: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal